
Hakata Clinic IRB
LOCATION
SOUSEIKAI Hakata Clinic (Fukuoka)
*Sponsors can also attend the IRB via web conference services. All studies conducted at SOUSEIKAI sites can be reviewed at our IRB.
DATE
Every 3rd Thursday.
SUBMISSION
10 days prior to the desired IRB Date. If submitted electronically, 7 days prior to each IRB.
*For our current IRB schedule, please see below.
RECEIPT OF RESULTS
Next Business Day.
IRB BOARD MEMBERS
Members include (male and female) physicians, legal attorneys, interpreters, business personnel, and academics.
- Summary
- Schedule
Summary
| Name | Hakata Clinic Institutional Review Board |
| Address | Random Square 5 – 7F 6-18, Tenyamachi, Hakata-ku Fukuoka 812-0025, Japan Tel: +81-92-283-7701 Fax: +81-92-271-3010 |
| Meeting Place | Random Square 4F, 6-18, Tenyamachi, Hakata-ku Fukuoka 812-0025 Japan (Sponsors can participate in IRB via TV conference from Sumida Hospital in Tokyo) |
Schedule
2025
| January | 16th |
|---|---|
| February | 6th , 27th |
| March | 21st |
| April | 10th |
| May | 8th , 29th |
| June | 19th |
| July | 10th , 31st |
| August | 28th |
| September | 18th |
| October | 9th , 30th |
| November | 20th |
| December | 18th |
2026
| January | 15th |
|---|---|
| February | 5th , 26th |
| March | 26th |
| April | 16th |
| May | 14th |
| June | 4th , 25th |
| July | 16th |
| August | 6th , 27th |
| September | 17th |
| October | 8th , 29th |
| November | 19th |
| December | 17th |
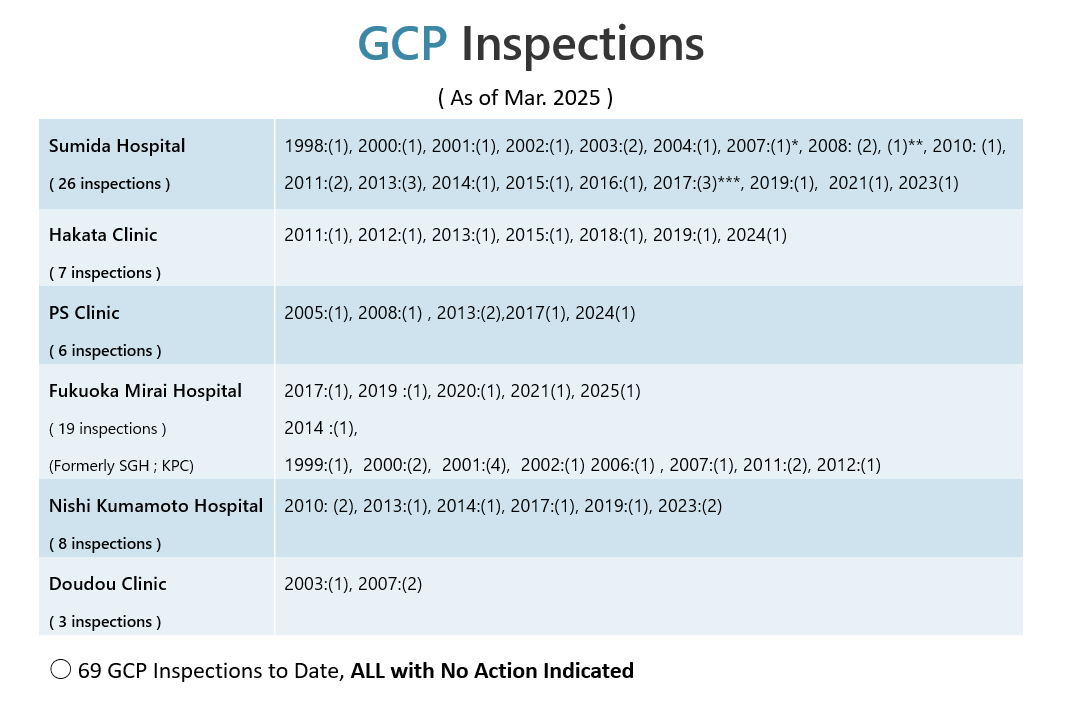
SGH : Sugioka Memorial Hospital
KPC : Kyushu Clinical Pharmacology Clinic
*MFDS : Ministry of Food and Drug Safety
**US FDA : US Food and Drug Administration
***MFDS and USFDA
